Pharmacovigilance
MedVigil offers bespoke pharmacovigilance services to our clients based on their requirements. Our team is committed to achieving your pharmacovigilance goals and meeting your deadlines. We will work with your operational procedures and workflows to integrate into your environment flawlessly.
ADVERSE EVENT REPORTING:
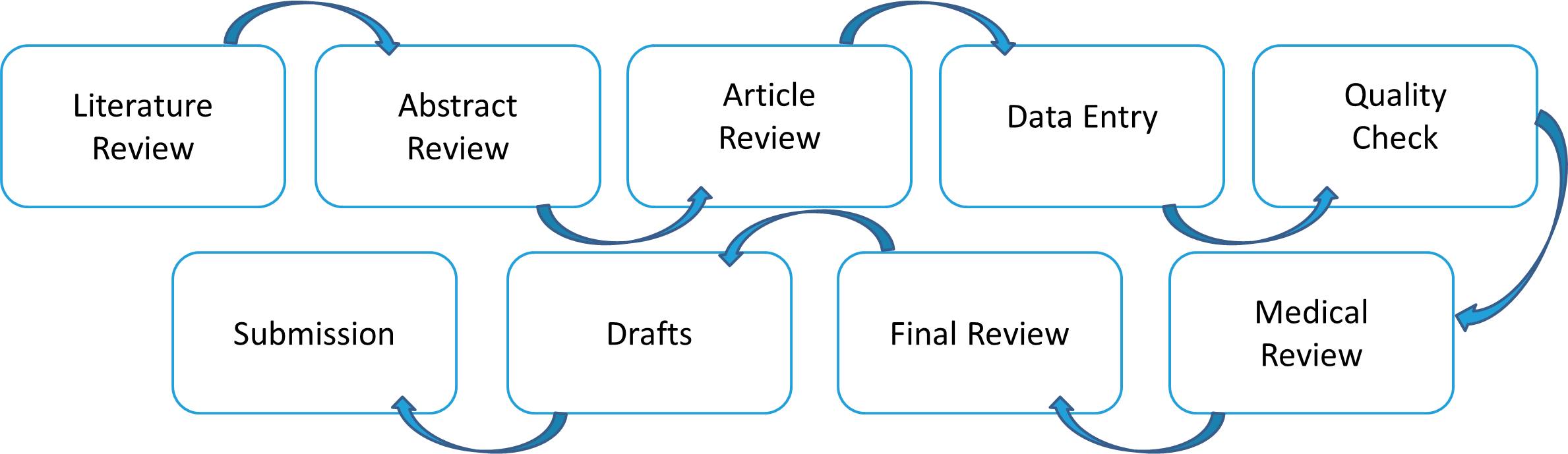
- MedVigil’s core service includes reporting adverse event reports. We receive adverse event experiences from all types of sources (clinical trials, solicited, spontaneous, literature, etc) from our client’s third party vendors in all types of formats. Once the information is received by our team it is immediately verified and entered into the drug safety database by drug safety experts.
- The information goes through an entire workflow cycle of assessment, triage, data entry, quality control and medical review before submission to the regulatory authority. We have an impeccable track record in management of the ICSR lifecycle. Our team will also generate and send follow up queries to the reporter in order to complete due diligence and collect all relevant safety data.
- Quality control measures have been put in place at every step of the cycle to ensure we meet highest quality standards. Our internal turnaround time for all cases whether serious or non serious is 24 hours from receipt of data so all submissions are on time.

Clinical Trials
Our highly skilled team of data entry associate and pharmacists perform case processing for large clinical trial cases. Their talent lies in sifting through large medical record data and extracting relevant information in the case as well as writing accurate medical narratives. The team also coordinates with the investigator and site for additional data requests and follow up information.

Post-Marketing
We handle all post-marketing case reporting right from spontaneous sources, solicited sources to social media sources.

Literature
We perform literature searches according to the client's schedule and do a thorough abstract review. Data entry from the full article and QC are performed by our literature team.

Special Scenario Cases
All type special scenario cases are handled by our team including but not limited to legal cases, pregnancy cases, product quality complaints, etc. We also perform ad hoc reporting projects as requested by client including poison center control reporting and data migration projects.
LITERATURE MANAGEMENT:
Literature screening process
- Medvigil has an in-house team of literature search specialists and a team that will help set literature search strings for each molecule. Weekly searches are performed on global literature databases in order to capture any adverse events related to our client molecules. Retrospective manual searches are run to make sure we have completed searches since the molecules approval date.
Literature assessment and case management
- Once a serious unexpected report is detected the case processing team will reassess the article and enter all the information into the safety database after which it will go through the product lifecycle and then be submitted to the FDA within the required timeline. The team will provide the client with a weekly or monthly literature report indicating number of abstracts and articles reviewed and number of cases generated. The team will also provide a CIOMS line listing of literature reports at the end of each month along with reconciliation.
SIGNAL DETECTION AND MANAGEMENT:
Timely detection of safety signals and their subsequent evaluation is essential in pharmacovigilance. Our team can identify and evaluate safety signals and provide expert advice for new concerns which might impact the benefit to risk ratio of the molecule.
AGGREGATE REPORTING:
Our scientific writing and pharmacovigilance teams in collaboration can support your aggregate reporting requirements for PADERs, PBRERs, PSURs and DSURs. We can also communicate with your existing case processing partner to provide quality aggregate reports. We can work on a report in full entirety or specific sections.

Periodic benefit/risk evaluation reports (PBRERs)

Periodic safety update reports (PSURs)

Developmental safety update reports (DSURs)

Periodic adverse drug experience reports (PADERs)

Support for risk management plans (RMPs) and risk evaluation and mitigation strategy (REMS)
DATA MIGRATION:
Data migration approach includes the migration plan that defines the overall do’s and don’ts of the activity. The migration plan is drafted in-line covering all the aspects required for the legacy data migration from the existing system (safety database) to a newer safety database, or migration of the legacy data into the existing system.
- Direct database to database migration
- Fully-automated database migration
- Semi-Automated database migration
- Manual database migration
We have vast expertise and an experienced team to handle the legacy data migration for clients across geographies. Well-established and in-depth procedures, work instructions and guidance are listed with end to end activity of handling the legacy data transfer from various safety databases. We have a predefined checklist to ensure that the relevant and appropriate data which need to be migrated are captured accurately.